- Home
- Extraneal Letter
Extraneal Letter
2 August 2024
Dear Patient,
Shortage of EXTRANEAL 7.5% w/v icodextrin peritoneal dialysis solution bag AHB5546 (AUST R 91344) and alternate supply arrangement under section 19A of the Therapeutic Goods Act 1989
This letter is to advise of an alternate supply arrangement for the Australian registered medicine, EXTRANEAL 7.5% w/v icodextrin peritoneal dialysis solution bag AHB5546 (AUST R 91344), sponsored by Vantive Pty Ltd (a legal entity of Baxter Healthcare).
In response to the short supply of the Australian registered medicine, Baxter has secured supply of an alternative product, EXTRANEAL Peritoneal Dialysis Solution with 7.5% Icodextrin 2000mL (Singapore), on a temporary basis. This product is NOT registered in Australia and supply is authorised by the Therapeutic Goods Administration (TGA) under section 19A of the Therapeutic Goods Act 1989 until 31 July 2025.
EXTRANEAL Peritoneal Dialysis Solution with 7.5% Icodextrin 2000mL (Singapore) is marketed and registered in Singapore and the packaging has labelling in English language. The section 19A product contains the same active ingredients and strength to the Australian registered product.
There are some minor differences in the way EXTRANEAL Peritoneal Dialysis Solution with 7.5% Icodextrin 2000mL (Singapore) is packaged compared to EXTRANEAL 7.5% w/v icodextrin peritoneal dialysis solution bag AHB5546 (AUST R 91344). These differences are as described below:
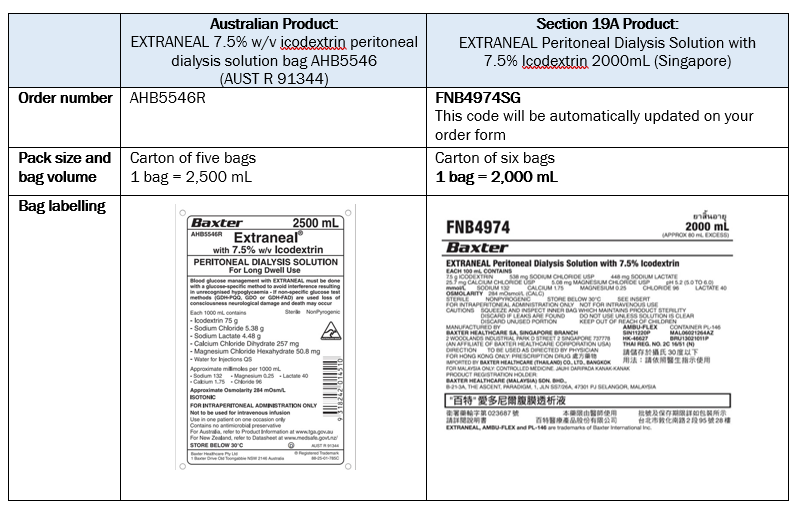


Note: If you have an Extraneal Last Fill volume of greater than 2,000 mL, please contact your dialysis unit or healthcare professional to discuss possible changes to your prescription.
Please refer to the product leaflet provided with your delivery of EXTRANEAL Peritoneal Dialysis Solution with 7.5% Icodextrin 2000mL (Singapore) for more information.
If you have any questions or concerns regarding the use of this alternative product, please contact your dialysis unit or healthcare professional.
Reminder - blood glucose management with Extraneal should be done with a glucose-specific method to avoid interference resulting in unrecognised hypoglycaemia. If non-specific glucose test methods or test strips are used, loss of consciousness, neurological damage and death may occur. Further details can be found in your Extraneal Patient Kit or at www.glucoyoursesafety.com.
Timing Of Alternate Supply
You will start to see EXTRANEAL Peritoneal Dialysis Solution with 7.5% Icodextrin 2000mL (Singapore), Product Code FNB4974SG in your deliveries in the coming weeks.
Adverse Event Reporting
Reporting any suspected adverse event is important for the continued monitoring of the safety of all medicines. Any adverse events which are experienced with the section 19A product, Extraneal FNB4974SG should be reported by healthcare professionals and patients to Baxter Healthcare on 1800 920 133 or email to
[email protected]. Alternatively, this information can be reported to the TGA at: https://www.tga.gov.au/reporting-problems.
We have contacted your healthcare team to advise them of this temporary change and we appreciate this change may cause some inconvenience. We are focused on securing the ongoing supply of Extraneal and appreciate your patience as we transition to this alternative supply.
For further information, please contact your dialysis unit or Baxter Healthcare on 1800 920 133.
Yours faithfully,
Matthew Bain
General Manager – Renal Care
Baxter Healthcare Australia